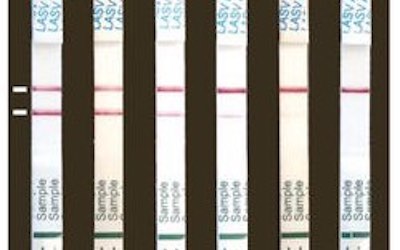
Corgenix, together with Tulane University, Autoimmune Technologies, Zalgen Labs, and other VHFC partners have received approval for their bedside 15-minute ReEBOV Ebola virus test. This will allow patients to be identified and cared for as quickly as possible. Previous Ebola tests required several hours, constant supplies of electricity, and highly trained staff to be performed, but the new test cuts that to minutes. The test directly detects viral particles and trials in West Africa suggest that it correctly identified 92% of patients.
Read full press release from Corgenix – Corgenix Receives FDA Authorization and WHO Listing for Emergency Use of Ebola Rapid Diagnostic Test
Read coverage by the UN Foundation – Testing The Test – A Scientist’s Journey In Search Of A Rapid Ebola Diagnostic
Read coverage by BBC – Fifteen-minute Ebola test approved