Viral hemorrhagic fevers
Lassa fever (Lassa) and Ebola virus disease (Ebola) are two of the most devastating human diseases. Infections with the causative agents – Lassa virus and Ebola virus – can lead to death in more than 70% of hospitalized patients. Unlike most viral hemorrhagic fevers, however, Lassa is not a rare disease that emerges as sporadic cases or in outbreak form. Although surveillance is inadequate to determine the true incidence, it is estimated that thousands of deaths from Lassa occur yearly across West Africa, where the disease is endemic (Figure 1). Lassa has the highest incidence of any viral hemorrhagic fever in the world, with the most cases in Nigeria and the “Mano River Union (MRU)” countries of Sierra Leone, Liberia, and Guinea. The number of Lassa cases, however, appears to be increasing in other parts of West Africa as well, with recent cases reported in Togo, Ghana, and Mali.
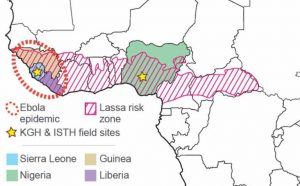
Figure 1. Overview of the Lassa endemic zone in West Africa.
Unlike Lassa, Ebola has typically been considered a rare and sporadic disease causing small-scale outbreaks in Central Africa. The Ebola epidemic that ravaged West Africa from 2013 to 2016, however, was unusual as it was found in a new area of the world, and with close to 30,000 cases, was by far the largest outbreak ever recorded. Weak healthcare infrastructure, overcrowded cities, community resistance, and a slow uncoordinated international response likely allowed the epidemic to spin out of control. Larger outbreaks have also since been observed in Central Africa, with a single outbreak in the DRC totaling thousands of cases.
Both Ebola virus and Lassa virus are classified as Biosafety level 4 (BSL-4) Category A agents with bioterrorism potential because of the severity of infection, the risk of aerosol-transmitted laboratory infections, and the lack of approved treatment options. Both viruses are also two of only eight pathogens that the World Health Organization considers to be of the highest priority, as they are “likely to cause severe outbreaks in the near future, and for which few or no medical countermeasures exist”. While more than 10,000 people died from Ebola during the West African epidemic, it is estimated that tens of thousands of individuals die from Lassa each year. These numbers are likely underestimated, as the healthcare infrastructure in the affected countries is extremely weak, surveillance almost non-existent, and most patients never present in the hospital.
Despite the high case fatality rates of hospitalized Ebola and Lassa patients, high seroprevalence rates in endemic areas suggest that human interaction with these viruses may be more frequent than generally acknowledged. Serosurveys performed by VHFC scientists to investigate population-wide exposures to Ebola virus and Lassa virus in West Africa have consistently found high levels of seropositivity to both viruses. The exposure rate for Lassa virus has been reported to be between 20-50%, whereas the level of exposure to Ebola virus – before the 2013-2016 Ebola epidemic – was estimated to be around 10%. These levels are much higher than one would expect for highly pathogenic viruses, but it is unclear whether these high rates are due to subclinical infections with Ebola virus and Lassa virus, false positives, infections with benign variants, or cross-reactivity with other viruses.
Subclinical infections have been reported for both Ebola virus and Lassa virus exposures, but the evidence is limited to anecdotal case reports or small-scale surveys. In addition, while ‘prototypical’ Ebola and Lassa are commonly associated with the development of hemorrhaging, this is a relatively infrequent clinical feature in most patients. Historically, mortality rates and clinical phenotypes have also varied tremendously for both Ebola and Lassa, suggesting that human and/or virus factors play key roles in determining clinical outcomes.
Why study viral hemorrhagic fevers?
Despite the severity of disease and suffering caused by both Ebola and Lassa, no effective drugs or treatments exist for these viruses. A recent VSV-based vaccine was FDA approved for Ebola, but no vaccine is currently available for Lassa and research is severely lacking. While Ribavirin is the drug of choice for treating Lassa virus infections, the drug is ineffective under most conditions and case fatalities remain high. Similarly, for Ebola virus infections no approved small molecule drugs currently exist, and while several drugs were tested during the 2013-2016 epidemic, none of them proved effective. More recently, therapies for Ebola based on monoclonal antibodies have shown promise and the development of more effective immune-based therapeutics is a area of active research for the VHFC.
Exacerbating the absence of countermeasures against Lassa and Ebola, a lack of robust field-deployable diagnostic assays has impeded our ability to rapidly and accurately diagnose patients with these diseases. This, combined with a limited understanding of how Ebola and Lassa viruses emerge and spread, means that infections with these viruses will likely increase and the risk of future outbreaks are high.
Our work with the VHFC
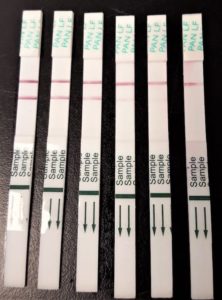
Figure 2. Example of our rapid diagnostic test for Lassa virus detection.
By establishing the VHFC – and it’s many sister consortia, including the Center for Viral Systems Biology, the Viral Hemorrhagic Fever Immunotherapeutics Consortium, and the Africa Centre Of Excellence for Genomics of Infectious Diseases – we aim to combat Ebola, Lassa and other emerging diseases by supporting research: (1) developing robust, fast, and accurate field-deployable diagnostics for these diseases; (2) developing novel drugs, including highly effective immunotherapeutics; (3) developing highly effective vaccines, providing long-term protection from infection with Lassa and Ebola, and (4) using genomics and ‘big data’ analyses to describe the transmission of these viruses and identify the factors underlying their spread across geographical regions.
Since the inception of VHFC in 2009, we have already made tremendous progress in achieving these goals. We have developed the first rapid diagnostics tests (Figure 2) for both Lassa and Ebola (commercially available from Zalgen), the latter of which was developed, manufactured, approved, and deployed within the first half of the Ebola epidemic in West Africa. During the 2013-2016 Ebola epidemic, as well as the ongoing Lassa epidemic, we have also used infectious disease genomics and high-throughput virus sequencing to show in detail how these viruses spread across West Africa. The insights from these studies have since been used to inform outbreak response and help mitigate the risk of future epidemics. Finally, as part of our ongoing work, we also have several highly promising drug and vaccine candidates for for Lassa and Ebola that we aim to advance to clinical trials within the next couple of years.


